FDA Gives Nutrition Facts and Supplement Facts Labels a Major Makeover After Two Decades
On May 27, 2016, the Food and Drug Administration (FDA) published in the Federal Register two final rules impacting the Nutrition Facts label: (1) "Food Labeling: Revision of the Nutrition and Supplement Fact Labels" (Nutrition Facts Rule)1 and (2) "Food Labeling: Serving Sizes of Foods that can Reasonably be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts; Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments" (Serving Size Rule).2
These final Nutrition Facts and Serving Size Rules mark the most significant changes to the Nutrition and Supplement Facts labels in more than 20 years, and will apply to all conventional foods and dietary supplements. (Meats, poultry, and processed egg products are not affected because labels for such products are regulated by the US Department of Agriculture). According to FDA, the update is intended to align labels with current data regarding health and nutrition, including findings from the Institute of Medicine (IOM) Dietary Reference Intakes Reports3 and the 2010 and 2015 Dietary Guidelines for Americans.4
The Nutrition Facts and Serving Size Rules take effect on July 26, 2016. Manufacturers with US$10 million or more in annual food sales must comply by July 26, 2018, while FDA gives manufacturers with less than US$10 million until July 26, 2019 to comply. FDA has indicated that it will issue guidance to clarify key aspects of the Nutrition Facts and Serving Size Rules as manufacturers work to conform to the Rules' terms.
This Advisory highlights a few significant changes to the Nutrition Facts label and related nutrition labeling requirements, as well as other FDA activity that should be on the radar for your company.
What's Different?
There are a few significant changes with respect to both the format and substance of future nutrition labeling that warrant special attention from all companies involved in the labeling of conventional food or dietary supplement products. Notable changes include a redesign of the Nutrition Facts label and new requirements for dual-column labeling, a mandatory declaration of added sugars, a narrower definition of dietary fibers, and new age categories for infants and children. These and additional key changes are discussed in more detail below.
New Design. While maintaining the "iconic" look of the Nutrition Facts label, the Nutrition Facts Rule makes several updates with the intent to give consumers access to information that they need to make informed eating decisions. The Agency prepared the following infographic summarizing the changes to the label:
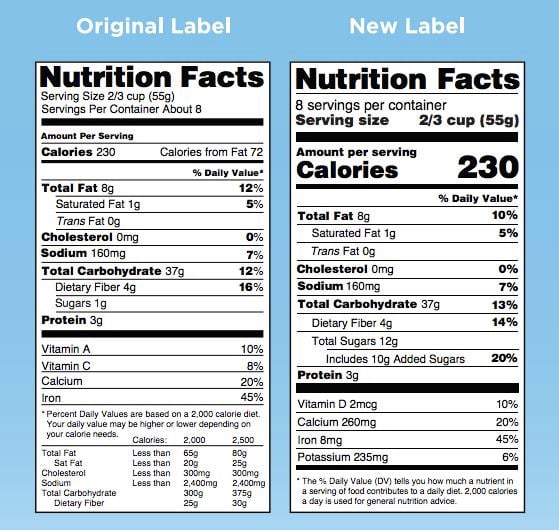
What's Different?, FDA (May 20, 2016)
Addition of Added Sugars. The Nutrition Facts Rule implements several changes aimed at helping consumers understand the amount of sugars added to food products. The current version of the Nutrition Facts label discloses the total amount of "sugars" contained in a serving, but does not require manufacturers to disclose the amount of "added sugars." In addition to requiring that the total grams of added sugars in a serving be disclosed, the final Nutrition Facts Rule establishes a Daily Reference Value (DRV) for added sugars and requires that the percent DV of added sugars be disclosed. If the amount of added sugars in a food is less than one gram per serving, then the label should state that the product is "Not a significant source of added sugars."
FDA's decision to include added sugars on the label was sparked by the 2015 Dietary Guidelines Advisory Committee's (DGAC) Scientific Report, which conducted a systematic review of the relationship between dietary patterns and health outcomes. The Scientific Report concluded that persons who consumed more than 10 percent of their total daily calories from added sugar were more likely to have difficulty meeting other nutritional requirements while staying within appropriate calorie limits. The Scientific Report also found that added sugars displaced other nutrients, and were found to contribute to overeating.
Based on the DGAC's findings, FDA concludes that added sugars are a "material" consideration for consumers in deciding what to eat. The final Nutrition Facts Rule requires that added sugars be disclosed, because consumers otherwise would have no way of knowing the amount of added sugars in a product. FDA recognizes that consumers may need help understanding how to interpret the new added sugars disclosure through a consumer education campaign.
"Mandatory" Vitamin D and Potassium Declaration. While the current label requires manufacturers to declare vitamin A, vitamin C, iron, and calcium on all food labels, the new Nutrition Facts Rule requires manufacturers to declare vitamin D, potassium, iron, and calcium on the Nutrition Facts label, functionally replacing vitamin A and vitamin C on the current label with vitamin D and potassium. Manufacturers can still voluntarily declare vitamin A and vitamin C, but will only have to declare them on the Nutrition Facts label in instances where the company makes claims about the nutrients or intentionally adds vitamin A or vitamin C to the food (the same standard applied to other vitamins and minerals for which Reference Daily Intake (RDI's) have been established).
Narrowed Definition of "Dietary Fiber." The final Nutrition Facts Rule defines "dietary fiber" as "non-digestible soluble and insoluble carbohydrates (with 3 or more monomeric units), and lignin that are intrinsic and intact in plants" and "isolated or synthetic non-digestible carbohydrates (with 3 or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health." The definition includes the two types of dietary fibers for which an authorized health claim exists—β-glucan soluble fiber and psyllium husk—as well as cellulose, guar gum, pectin, locust bean gum, and hydroxylpropylmethylcellulose.
The Agency intends to publish a separate notice to seek comments and evaluate the available scientific data on non-digestible carbohydrates, including inulin, bamboo fiber, soy fiber, pea fiber, wheat fiber, cotton seed fiber, sugar cane fiber, sugar beet fiber, and oat fiber. Additionally, companies may petition the FDA to amend its definition of dietary fiber to include additional fibers. FDA plans to issue a guidance document on the information that it recommends companies provide to the Agency for the scientific review and the approach it intends to take to evaluate the studies.
Clarification of Folate and Folic Acid Terms. The Nutrition Facts Rule makes significant changes to the terms that manufacturers may use to declare folate and folic acid in their conventional foods and dietary supplements. Under FDA's preexisting regulations, "folic acid" and "folacin" could be used as synonyms for "folate" on the Nutrition Facts or Supplemental Facts labels. The rule removes "folacin" from the list of synonyms that may be used for "folate" in the Nutrition Facts and Supplemental Facts labels. It also removes the term "folic acid" from the list of synonyms that may be added in the parentheses immediately following "folate" on the Nutrition Facts label or in the place of "folate" on the Supplemental Facts label. The rule requires that, when declared, labels must include both the terms "folate" and "folic acid" on the Nutrition and Supplemental Facts labels.
Redefined Single-Serving Containers. FDA also addresses its position that package sizes influence the amount of food consumed by expanding the range of packages that must be labeled as single-serving products.
Currently, when the percent of the Reference Amounts Customarily Consumed (RACC) is between 150 and 200 percent (one or two servings), manufacturers can decide whether to label the container or unit as a single-serving product. The final Serving Size Rule eliminates this flexibility and requires a single-serving label for containers or units with less than 200 percent of the RACC (e.g., 20-ounce soda). FDA also allows manufacturers to voluntarily use a dual-column label for products between 150 and 200 percent of the RACC, with the aim that consumers will compare the nutrition information to that of similar products.
Additionally, the final Serving Size Rule allows manufacturers to voluntarily use a dual-column label on single-serving products with "nutrition information per unit of a product that is in discrete units." This would apply to products with multiple individually wrapped units in a single container, for example.
<p">If a product contains 200 percent or up to 300 percent of the RACC (a product that consumers eat in one or multiple sittings), then FDA requires dual-column labeling of quantitative amounts and percent DVs for a single-serving size and the entire container. FDA explains that "[c]onsumption data show that while some people eat certain products in a single eating occasion, others eat the product over time or share it." The Agency believes that dual-column labeling will address all of these eating occasions.</p">The upper limit of 300 percent is a change from the proposed Serving Size Rule, which placed the limit at 400 percent. FDA agreed with comments suggesting that more than 90 percent of food products have consumption levels at 300 percent or less of the RACC. FDA also noted that there could be unintended consequences for dual-column labeling at 400 percent, including "requiring dual-column labels on packages for which data shows people do not reasonably consume in a single eating occasion, such as a quart of milk [or] a 32 fl oz bottle of juice . . . ."
Updated Reference Amounts Customarily Consumed (RACC). FDA updates, modifies, and establishes RACCs in the Serving Size Rule, including increasing the RACC for ice cream from 1/2 cup to 2/3 cup, against the requests of many stakeholders. FDA bases its revision to the RACC on consumption data showing the median consumption of products in the new product category of "[i]ce cream, frozen yogurt, sherbet, frozen flavored and sweetened ice, frozen fruit juice: all types build and novelties" is about 2/3 of a cup.
Additionally, FDA reduces the RACC for all forms of yogurt, including drinkable yogurt, to 170 grams (6 ounces). The final Serving Size Rule also establishes a new serving size of "1 unit" for breath mints and keeps 2 grams as the reference amount for the product category "[h]ard candies, breath mints."
Labeling of Foods of Infants, Young Children, and Pregnant or Lactating Women. FDA replaces the current age category for infants and children under the age of 4 with two categories: (1) infants 7 through 12 months; and (2) children 1 through 3 years of age. The final rule changes the age category to be consistent with the age ranges included in the IOM Report and the Institute's age-specific DRI recommendations. The Agency believes the IOM's age ranges reflect the differences in growth and development between infants and young children. Manufacturers will still be required to label food products for infants and children under the age of 4, but FDA is not setting DVs for infants less than 7 months.
In addition to other statutorily required nutrients, the final rule mandates the declaration of calories for saturated fat and cholesterol for infants and children 1 through 3 years of age. The Agency notes that the declaration of calories for saturated fat and cholesterol provides more information to the consumers to make better, more informative decisions about their health and the health of children. While FDA acknowledges that total fat should not be limited for children under the age of 2, unless medically advised, the Agency plans to monitor fat and cholesterol intakes for the two new age groups and may revisit the labeling requirements for them in the future.
For the percent DV declaration, FDA maintains the mandatory declaration for protein on the Nutrition Facts label for infants and children 1 through 3 years of age.
Record Keeping Requirement. The Nutrition Facts Rule requires manufacturers to maintain records to verify mandatory declarations of added sugars, dietary fiber, soluble fiber, insoluble fiber, vitamin E, folate, and folic acid. Some comments to FDA's proposed Nutrition Facts Rule raised concerns that the Agency does not have the authority to require recordkeeping for nutrition labeling. FDA disagrees by explaining that recordkeeping would be limited to foods where a reliable analytical method is not available to the Agency. FDA notes that in some cases manufacturers only have access to this information.
What's Next?
More FDA Action Related to Nutrition Labeling. After years of class action litigation and looming GMO legislation forcing manufacturers to primarily focus their attention elsewhere, the release of the Nutrition Facts and Serving Size Rules, in some ways, reflects one of many steps FDA has taken this year to re-establish its voice as the authority with respects to what can and cannot be said about a food.
For example, earlier this month, FDA announced its intent to reevaluate its regulation of nutrient content claims, in part due to a citizen opinion filed by KIND LLC regarding FDA's requirements for labeling a product as "healthy." Under FDA's current regulations, food may be labeled as "healthy" if it meets set criteria based on the amount of fat, saturated fat, cholesterol, and other nutrients in the food. In a Citizen Petition to the FDA in December 2015, KIND LLC pointed to recent research, including evidence discussed in the 2015 DGAC Scientific Report, supporting focus on the overall nutritional quality of foods rather than the specific nutrient levels in foods.5 For example, the petition points out that while nuts may be high in fat, nut intake is associated with several health benefits and a reduction in all-cause mortality. In response, earlier this month, FDA announced that it will solicit comments in the "near future" to reevaluate regulations concerning nutrient content claims and the use of the "healthy" on food labeling.6
Last week, the Agency released updated guidance affirming its 2009 draft guidance advising against the use of the term "evaporated cane juice" to declare the presence of sweeteners derived from the fluid extract of sugar cane. It is reasonable to expect this FDA action to increase the number of class action complaints related to the use of "evaporated cane juice" as the common and usual name for sugar in product labeling. By the end of the year, FDA is also expected to opine on another labeling issue that could have a direct impact on consumer litigation—the use of "natural" on food labels. FDA formally closed the docket on its November 2015 request for comment regarding the use of "natural" on food labels on May 10 and could take action related to "natural" this year as well.7
Manufacturers should also anticipate that FDA's overhaul of its Nutrition Facts label will be the first of many actions by the Agency to update its food labeling policies. The final Nutrition Facts Rule notes that in future rulemakings, the Agency plans to evaluate claim regulations and changes to eligibility for claims based on the Nutrition Facts and Serving Size Rules.
Consumer Education. Stakeholders, including the Grocery Manufacturers Association, are calling for a "robust consumer education effort" to help prevent confusion caused by the final Nutrition Facts Rule's numerous changes.8 In the final Nutrition Facts Rule, FDA agreed that a consumer education campaign will be critical to making the new food label a successful tool that helps consumers make healthy food and beverage choices. The Agency plans to work closely with federal agencies, state health departments, and other stakeholders to develop and disseminate its new educational materials.
Among its intended focus areas, FDA recognizes that particular attention is needed to help consumers understand how to appropriately interpret the new added sugar disclosure. Stakeholders have raised the concern that consumers may mistakenly believe that added sugars should be entirely avoided. The rule reiterates that FDA recognizes that, in moderation, foods with added sugars may be part of a balanced diet. The campaign will focus on helping consumers understand the appropriate weight to place on added sugars, and how foods with added sugars may be included without overlooking other parts of a healthy diet.
Consumer Advocacy and Litigation. Manufacturers should also anticipate increasing pressure from consumers and public health advocates related to claims regarding the healthfulness of their food products. The sweeping changes to FDA's food labeling policies may result in legal challenges against manufacturers centered on nutrient content claims or their compliance with the new Nutrition Facts and Serving Size Rules.
-
81 Fed. Reg. 33742 (May 27, 2016).
-
81 Fed. Reg. 3400 (May 27, 2016).
-
Dietary Reference Intakes Tables and Application, National Academy of Science.
-
Dietary Guidelines for Americans, 2010; Scientific Report of the 2015 Dietary Guidelines Advisory Committee (February 2015).
-
Citizen Petition, KIND Snacks (December 1, 2015).
-
Statement on FDA's Actions on Labeling of KIND Products, FDA (May 10, 2016). Two days later, FDA reinforced its focus on what can and cannot be said for food products, by issuing an updated guidance document regarding "medical foods." See "Guidance for Industry: Frequently Asked Questions About Medical Foods; Second Edition." Among other things, the guidance document provides helpful insight into FDA’s narrow definition for the category as well as its position that diabetes and pregnancy are not conditions for which a "medical food" can be marketed.
-
What is the meaning of 'natural' on the label of food?, FDA; Use of the Term "Natural" in the Labeling of Human Food Products; Request for Information and Comments, FDA, 80 Fed. Reg. 69905 (Nov. 12, 2015); and Use of the Term "Natural" in the Labeling of Human Food Products; Request for Information and Comments; Extension of Comment Period, FDA, 80 Fed. Reg. 80718 (Dec. 28, 2015).
-
GMA Statement on Nutrition Facts Panel Revision (May 20, 2016).


